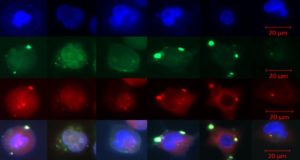
Genetic researchers at Christiana Care Health System’s Gene Editing Institute have demonstrated that short pieces of synthetic single-stranded DNA, known as oligonucleotides, when used in gene editing with the CRISPR/Cas9 technique, can promote the repair of genetic mutations, help achieve a cleaner “cut” of the gene and reduce the degree of genetic fraying, or heterogeneity, that occurs during gene editing.
Published in the Sept. 9 issue of the journal Scientific Reports, part of the Nature Publishing Group, the research shows that oligonucleotides can act to hold together the ends of the cut DNA and reduce heterogeneity. The article, titled “Analyses of Point Mutation Repair and Allelic Heterogeneity Generated by CRISPR/Cas9 and Single-Stranded DNA Oligonucleotides,” also maps in new detail, through high-level bioinformatics, what takes place in each part of the gene when a CRISPR cut is made.
“The applications for this in genetic and cancer research are vast,” said world-renowned molecular biologist and gene-editing pioneer Eric Kmiec, Ph.D., lead author of the study and director of the Gene Editing Institute at the Center for Translational Cancer Research at Christiana Care’s Helen F. Graham Cancer Center & Research Institute. “If you want to repair a genetic mutation, you can’t allow the DNA to be ripped apart when CRISPR makes a cut. You need it to remain intact to execute the repair, and that’s what the oligonucleotide enables us to do.”
Gene editing can be thought of as analogous to a word processor’s spell-check and cut-and-paste functions, he said. “First you have to locate, cut and excise the genetic base, or letter. That’s what the CRISPR does. Then the oligonucleotide enables us to repair and insert the right base.”
Dr. Kmiec also used the analogies of cutting ribbons and applying bandages to describe the effect of the oligonucleotide. “CRISPR can leave frayed ends in the DNA,” he explained. “It’s as though you’ve tried to tear a ribbon in two. But for gene therapy, you want a clean cut at a precise spot with minimal fraying, like cutting a ribbon with scissors, so that the ends can re-close. That’s what the oligonucleotide does. It also helps to maintain the cut, like using a bandage to hold the ends together. Without the oligonucleotide, the ends fray and we lose the gene.”
Christiana Care’s Gene Editing Institute, which recently entered a partnership with Philadelphia’s Wistar Institute to accelerate breakthrough cancer research in the human genome, has been studying the gene editing mechanism for many years in the hope of fixing the genetic mutations that cause disease. In his work, Dr. Kmiec noticed that whenever he and his researchers used the single-stranded DNA molecule, the oligonucleotide, the result was cleaner, and the gene came out healthier and nearly intact. That led them to the discovery that the oligonucleotide was not only helping to repair the mutation but was also preventing the destruction of the gene from fraying or tearing.
“We were studying sickle-cell anemia at the time, and this was a side-bar observation,” said Dr. Kmiec. “But we followed up on it and found that the combination of the CRISPR and the oligonucleotide was doing something that is much more widely applicable for anyone doing this kind of work.”
The next step for the research team will be to move from laboratory model cells to human progenitor cells. A federal biosafety and ethics panel recently approved the first use of the CRISPR/Cas9 technique in human patients, and Dr. Kmiec looks forward to that stage in his own research.
“That’s exactly where all of us in this field are headed,” said Dr. Kmiec, “and Christiana Care will be right there on the playing field.”
Along with Dr. Kmiec, authors of the study include Pawel Bialk at the Gene Editing Institute, Brett Sansbury at the Delaware State University Department of Chemistry, Natalia Rivera-Torres at the University of Delaware Department of Medical Laboratory Science, and Kevin Bloh at the Gene Editing Institute and the Nemours Center for Childhood Cancer Research.
The Gene Editing Institute at the Graham Cancer Center is a worldwide leader in personalized genetic medicine. Founded and led by Dr. Kmiec, the Gene Editing Institute is unlocking the genetic mechanisms that drive cancer and that can lead to new therapies and pharmaceuticals to revolutionize cancer treatment. The Gene Editing Institute also provides instruction in the design and implementation of these precise new genetic tools.
The Helen F. Graham Cancer Center & Research Institute, a National Cancer Institute Community Oncology Research Program, is part of Christiana Care Health System. With more than 219,000 patient visits last year, the Graham Cancer Center is recognized as a national model for multidisciplinary cancer care and a top enroller in U.S. clinical research trials. Its Gene Editing Institute, Center for Translational Cancer Research, Tissue Procurement Center, statewide High-Risk Family Cancer Registry and collaborations with world-renowned scientists at facilities such as the University of Delaware and The Wistar Institute in Philadelphia are opening new avenues to more quickly translate cancer science into cancer medicine.