Scientists have developed an affordable, downloadable app that scans for potential unintended mistakes when CRISPR is used to repair mutations that cause disease. The app reveals potentially risky DNA alterations that could impede efforts to safely use CRISPR to correct mutations in conditions like sickle cell disease and cystic fibrosis. The development of the new tool, called DECODR (which stands for Deconvolution of Complex DNA Repair), was reported today in The CRISPR Journal by researchers from ChristianaCare’s Gene Editing Institute.
“Our research has shown that when CRISPR is used to repair a gene, it also can introduce a variety of subtle changes to DNA near the site of the repair,” said Eric Kmiec, Ph.D., director of ChristianaCare’s Gene Editing Institute and the principal author of the study. “We developed DECODR to accelerate the development of CRISPR gene therapies by providing a way to rapidly detect these changes so we can determine whether they pose a risk to patients.”
He said that such changes may be harmless, but researchers must determine whether the changes can disrupt the repair itself or alter gene function in a way that could potentially harm patients. He said an easy-to-use tool like DECODR also will be valuable for assessing whether CRISPR gene repairs produce different outcomes from patient to patient that affect safety and efficacy.
“Like many medical interventions, CRISPR gene therapies are likely to come with a mix of risks and benefits,” he said. “More information is needed to ensure patients can make an informed decision.”
Intern’s inspiration leads to potentially industry-changing new tool

The development of DECODR began in 2019 when a young intern at the Gene Editing Institute with a talent for software development offered to help researchers develop an app for analyzing the huge volumes of DNA data produced by a single CRISPR edit.
Rohan Kanchana said he was intrigued by the fact that the best way to screen large populations of cells, a process known as “targeted deep sequencing,” was too costly and time consuming to be practical for most labs. Meanwhile, his mentors noted that available alternatives to deep sequencing did not detect the full range of DNA mutations that may be introduced by a CRISPR gene repair.
The study in The CRISPR Journal presents evidence that the DECODR app, which was written with open-source software to allow for easy updating, can essentially produce the same data as a deep sequencing process in much less time and at a fraction of the cost.
The study reports that DECODR can accurately determine a wide range of insertions and deletions of DNA code that may occur during a CRISPR-directed gene repair to better determine how a CRISPR experiment impacts a targeted gene of interest.

“We were particularly interested in developing an algorithm that is capable of crunching a large amount of data to break down or ‘deconvolute’ the outcome of repairs that involve deleting and inserting long strands of DNA code,” said lead author Kevin Bloh, a researcher at the Gene Editing Institute and a Ph.D. candidate in medical and molecular sciences at the University of Delaware.
“These are edits where there is a greater risk that unintended mutations introduced by CRISPR could disrupt the targeted gene.”
“The beauty of DECODR is that it is designed to be scaled to evaluate insertion and deletions of DNA executed by a CRISPR edit regardless of size,” added Byung-Chun Yoo, Ph.D., study author and associate director of the Gene Editing Institute.
“This means it can evolve as researchers develop the capacity to attempt more complex repairs.”
The challenge of unintended consequences in CRISPR
CRISPR stands for “clustered regularly interspaced short palindromic repeats.” It is a defense mechanism found in bacteria that can recognize and slice up the DNA of invading viruses. Scientists have learned how to modify this mechanism so it can be directed to “edit” specific sequences of DNA code, with a focus on repairing DNA mutations that cause deadly diseases.
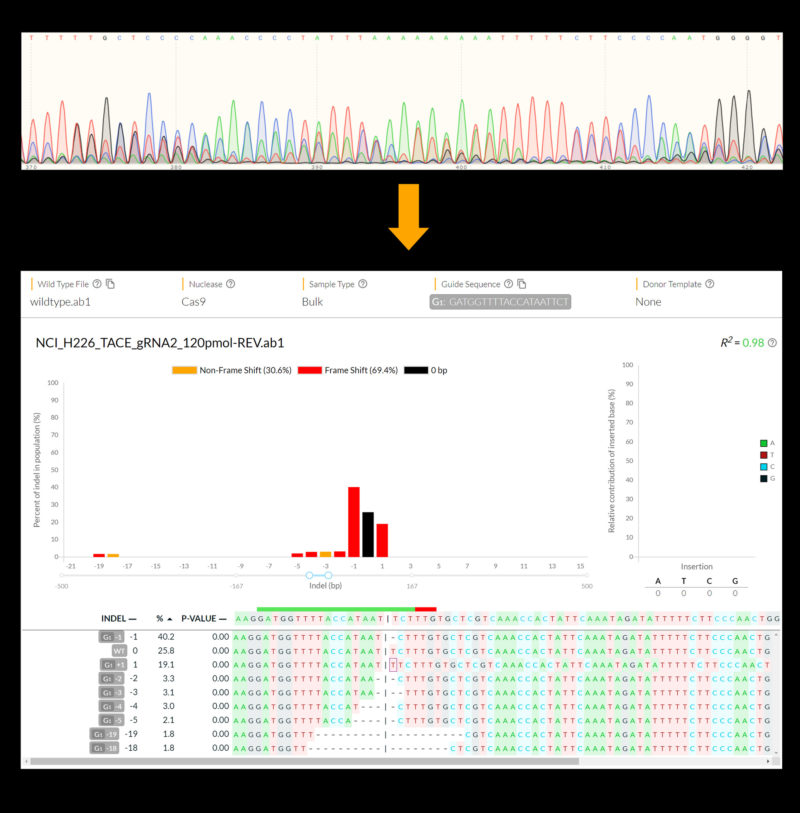
Dr. Kmiec said DECODR is focused on CRISPR edits that delete a strand of DNA code that is causing a gene to malfunction and insert a new strand of code that corrects the problem—as opposed to edits that are focused on just eliminating or “knocking out” a gene. He noted that even though the repair may target a single gene—such as the malfunctioning gene that causes sickle cell patients to produce abnormal red blood cells—that gene is present in a large number of cells. And he said there is evidence that the process of deleting and inserting DNA code has the potential to introduce subtle mutations near the site of the gene repair, mutations that may vary from cell to cell.

Last year, in a study in the Nature journal Communications Biology, scientists at the Gene Editing Institute found that such unintended changes are more common than previously understood.
According to the new study, “it is the unpredictability and diversity of edited outcomes within a population of cells that have raised caution as CRISPR-directed gene editing programs advance toward clinical application.”
Kmiec pointed out that both studies are focused on changes introduced by CRISPR around the site of the intended repair, not on the separate concern about the risk of CRISPR causing “off-target” mutations by drifting far afield from the target and making random cuts across the genome.
For now, Kmiec said DECODR is currently intended solely as a research tool, not in the clinical evaluation of individual patients. It is available online at decodr.org/analyze as a free version that researchers can immediately use to evaluate the outcomes of their CRISPR gene editing experiments. The Gene Editing Institute also is in discussion with partners about licensing a commercial version of DECODR that could provide researchers with a wider range of options.
Learn more about ChristianaCare’s Gene Editing Institute.