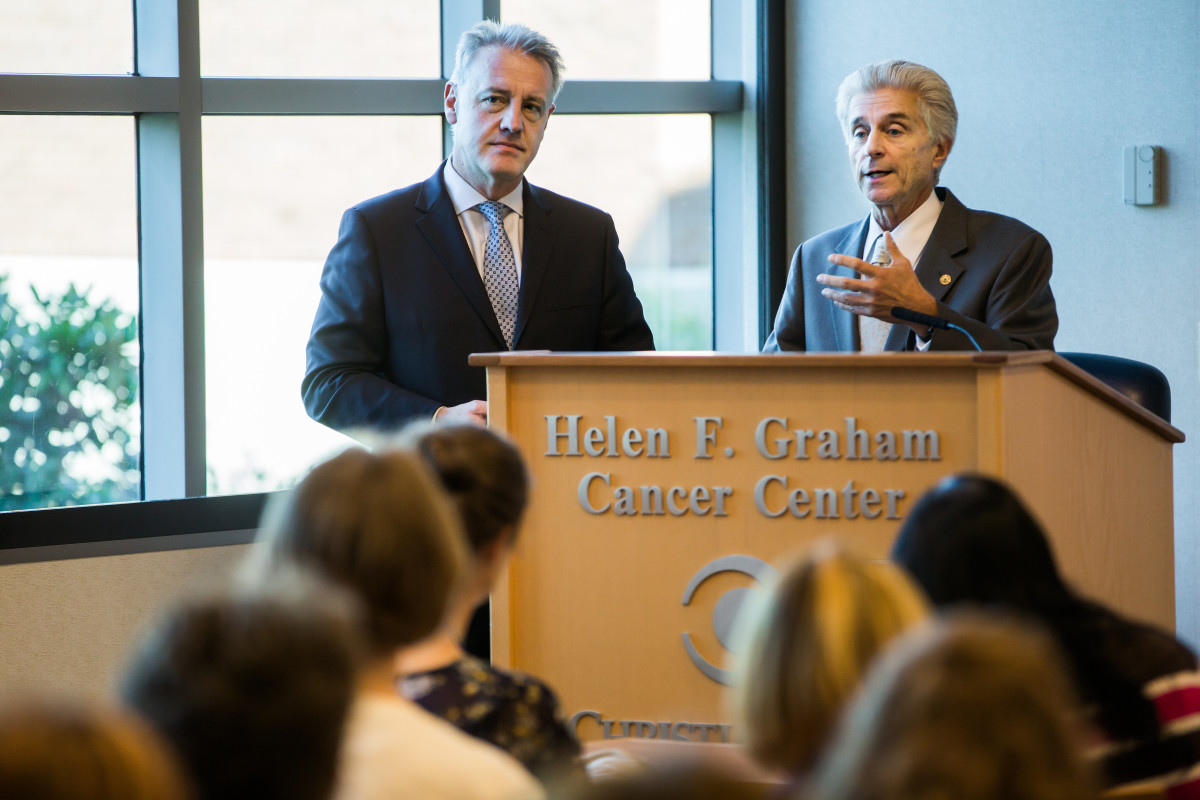Christiana Care’s Helen F. Graham Cancer Center & Research Institute and The Wistar Institute in Philadelphia will pursue the next phase of their historic four-year partnership by applying to become a National Cancer Institute (NCI) designated Cancer Center. Wistar has been NCI-designated since 1972.
On Nov. 19, Nicholas J. Petrelli, M.D., Bank of America endowed medical director of the Graham Cancer Center, and Dario C. Altieri, M.D, director of the Wistar Cancer Center and president and chief executive officer of the Wistar Institute, will make a formal presentation to NCI as part of the NCI designation application. If the status is granted in 2018 following on-site program evaluations, the Graham Cancer Center and Wistar will be recognized as partners with a cohesive research agenda and defined clinical goals.

“At the moment there is no cancer consortium program between an NCI-designated research institute, such as Wistar, and an NCI-selected community center, such as Christiana Care. We would be the first,” said Dr. Petrelli, who joined Dr. Altieri in a presentation on the partnership at the Graham Cancer Center on Oct. 16.
To earn designation, NCI guidelines call for a formal written agreement to ensure stability, as well as integrated research, as evidenced by a history of collaboration, including joint grants and publications. Shared fundraising and a unified institutional review board are also encouraged. In addition, each is expected to hold a portfolio of peer-reviewed cancer-related research grants that contribute to the center’s scientific goals.
“Among our accomplishments already are a demonstrated history of collaboration on grants, research, fundraising and publications,” said Dr. Petrelli. “Wistar is an international leader in biomedical research, and the initiatives between our two institutions will only grow. That means more innovative cancer research leading to better cancer therapies for our patients. Our collaboration will also continue to provide outstanding opportunities for our scientists in the Center for Translational Cancer Research to work with Wistar scientists on specific translational oncology research projects.”

Dr. Petrelli explained that the application builds on the Graham Cancer Center having been selected in 2007 as an original NCI Community Cancer Centers Program (NCCCP), putting Christiana Care into a network of centers capable of bringing the latest treatments to patients. Today, the Graham Cancer Center is a model for cancer treatment, research and supportive care, reaching 3,200 new patients a year.
Dr. Petrelli outlined other achievements and assets that Christiana Care brings to the partnership, including:
- Since 2002, Christiana Care has built a high-risk family cancer registry and hired five full-time genetic counselors. The registry is a national leader with 6,500 families registered.
- Multidisciplinary disease-specific treatment centers (MDCs) offer patients face-to-face conferences with a team of disease specialists to begin therapy, all in one visit. MDCs have greatly enhanced patient satisfaction.
- The NCI-funded Community Oncology Research Program (NCORP) at the Graham Cancer Center is one of the top enrollers in cancer clinical trials in the United States.
- The Graham Cancer Center has a record of improving early-detection of cancers and working successfully to reduce cancer mortality in Delaware. The mortality rate for men and women is dropping twice as fast as the national average.
- A robust pharmaceutical trials program is conducting Phase I and Phase II drug trials in collaboration with research centers across the country. The studies often give patients the opportunity to be part of the newest cancer treatments.
- The Tissue Procurement Center has provided research-quality specimens for the groundbreaking Cancer Genome Atlas Project since 2009, supporting the work of scientists to unlock cancer’s vulnerabilities. The center also processes biological specimens for Wistar for melanoma, ovarian, and head and neck cancer research, as well as the development of a commercially viable blood test for the most commonly diagnosed from of lung cancer, known as non-small cell lung cancer.
The NCI application also takes advantage of the Wistar Institute being a private, nonprofit basic-science institute with 30 laboratories and distinguished achievements in vaccine development, having created standard-of-care protections against rubella, rabies and rotavirus. The vaccinations earned $17.7 million in licensing revenues in 2013. Wistar was designated an NCI cancer center in 1972, focusing research on understanding the causes and treatment of cancer. The institute’s cancer center has a history of significant advances in cancer genetics, cancer biology, tumor immunology and virology.
Since 2011, the growing institutional partnership has combined Wistar’s strengths in basic biomedical research with the Graham Cancer Center’s exceptional cancer treatment of patients. One goal of the growing Christian Care–Wistar collaboration has been to translate or advance research discoveries made in Wistar’s labs into early phase (phase I and II) clinical trials with patients at the Graham Cancer Center. At 20 percent, the Graham Cancer Center has had one of the nation’s highest patient accrual rates into cancer clinical trials, far above the national average of 4 percent.
“With our Christiana Care collaboration we are bringing our research to the next level, which is into the community where 85 percent of oncology care is given,” said Dr. Altieri. “At Wistar, we would like to create opportunities for innovation and partnership with Christiana Care at the cutting edge of basic and translational research, where we see a real need.”