Christiana Care’s Helen F. Graham Cancer Center & Research Institute has established the Gene Editing Institute at the Center for Translational Cancer Research (CTCR) under the direction of world-renowned molecular biologist and gene-editing pioneer Eric Kmiec, Ph.D.
“The installation of the Gene Editing Institute under the direction of Dr. Kmiec at the CTCR places our translational science program on equal footing with the very best in the nation,” said Nicholas J. Petrelli, M.D., Bank of America endowed medical director of the Helen F. Graham Cancer Center & Research Institute. “Bringing together scientists such as Dr. Kmiec and his team with our clinicians under one roof promises to be the catalyst that will skyrocket progress toward personalized genetic medicine for our patients.”
This could change everything from the way we develop treatments to how we impact patients.
The CTCR’s Gene Editing Institute is dedicated to education, technology development and scientific research into the very core of the human genome. This means designing the tools scientists need to more easily and efficiently manipulate and alter human genetic material, and to better understand and cure many genetic diseases, including cancer.
“Only in the last four or five years have scientists succeeded in putting together a genetic toolbox for us to manipulate and control the genetic material in human cells for therapeutic purposes,” Dr. Kmiec said. “This could change everything from the way we develop treatments to how we impact patients.”
Surgery on our genes?
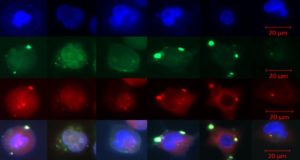
Among the most important tools used in gene editing are designer proteins capable of honing genetic material into shears used to disrupt and repair rogue genes at work in many disease processes. With names like ZFNs, TALENs and CRISPRs, these are what scientists call programmable nucleases, genetically engineered in the lab from plants and bacteria, that enable researchers to essentially perform microsurgery on genes. They act like molecular scissors to precisely cut into and delete, add to or modify a particular faulty strand of DNA to restore it to normal, or to test new, targeted drugs.
Scientists learned how to make these gene-editing tools by studying how bacteria defend against attacking viruses and nature’s own programming for cell repair.
Among the newest tools in the box and currently in demand are CRISPRs, short for “clustered regularly interspaced short palindromic repeats.” Some scientists have hailed CRISPRs as the biggest biotech discovery of the century, because they give scientists the ability to quickly and simultaneously make multiple genetic changes to a cell.
Dr. Kmiec says that although CRISPRs are easier to program and work more efficiently than the other models, they have a tendency (about 2 percent of the time) to cut off-target at sites other than those intended, a process called off-site mutagenesis.
“If you are designing treatment for an inherited disease like sickle cell anemia, for example, where targeted genes are surrounded by a family of close cousins, mistaking the target could be very serious and lead to permeant unwanted genetic defects,” he said.

Gene editing to repair and prevent disease
Dr. Kmiec’s pioneering research on sickle cell anemia has led to research and development of the next generation of CRISPRs and TALENs, and to even more promising variations such as singlestranded DNA oligodeoxynucleotides (ssODNs) to improve precision and reliability. In their largest research project to date, Dr. Kmiec’s team is perfecting these tools to study chromosomal translocations — what happens when bits of chromosomal DNA swap places in a cell, which is known to set off the chain of events leading to several types of leukemia.
“We are using CRISPRs to make the translocations in normal cells and changing them into leukemia cells in our lab,” he explained. “This allows us to study in real time how the cell adapts, stage by stage, to that translocation event. In this way we hope to create a series of snapshots of how cancer progresses, and to model these events one by one.”
Although his team is focused on studying blood cell cancers at present, Dr. Kmiec said he plans ultimately to study chromosomal translocation factors in solid tumor cancers as well.
“This work will probably be a major focus of our research over the next 10 years,” he said.
The ability to map a cell’s journey toward cancer would be a treasure trove for cancer drug development. Using such an atlas, scientists could change or disable target genes at any point along the cancer continuum to screen anti-cancer drugs for maximum therapeutic benefit. He noted that this strategy could also be used to establish tester cell lines that contain genotypes from individualized patients who represent the most common genetic backgrounds of a particular cancer. To this point, most of the work on human cells using gene-editing tools has been done in the lab or in small pre-clinical studies, but the potential and advantage for therapeutic development is clear and emerging.
“While setbacks are naturally expected, with enough momentum, coupled with robust science, investigators will push toward making significant and practical contributions to cancer and other disease research using these programmable nucleases,” he said.
The challenge centers on the complexity of the human genome. “Clearly, caution must be taken in assuming that these tools operate and act at only one site throughout the genome. Off-site mutagenesis is of particular concern to those of us who are developing these tools for clinical application.”
If scientists can perfect the delivery system of these high potential gene-editing tools, Dr. Kmiec predicts they will open the door to a whole new age of molecular medicine.
Technology development and customized services
The CTCR Gene Editing Institute will also design and manufacture gene-editing tools on-demand for outside research projects and teach other researchers how to design and use the tools effectively.
“What distinguishes the Gene Editing Institute is our ability to offer clients assistance in developing their experimental protocols and to select the appropriate gene-editing tools that will work best to achieve their research goals,” Dr. Kmiec said. “Our discovery work puts us at the forefront of this sophisticated technology, and we are willing to share our expertise to model these tools for our clients.”
The client list extends as far as the University of Hawaii, the University of North Dakota and Dartmouth College in New Hampshire, as well as to our own region at The Wistar Institute in Philadelphia, Delaware State University and Nemours/ Alfred I. duPont Hospital for Children. In-house, scientists at the Helen F. Graham Cancer Center & Research Institute are using TALENs to study BRCA1 gene activity in breast cancer and CRISPRs to study cancer stem cell function.
The Gene Editing Institute also offers on-site workshops and webinars to train scientists, students and faculty in gene editing methodologies. A summer student internship program is under development, and a project is under way with commercial biomedical engineering and educational partners on a kit to help teach gene-editing techniques to students in laboratory courses at universities, community colleges and high schools. The kit models the work done at the CTCR to develop and test gene-editing systems.
“During the course of a two-week lab assignment, students can use a CRISPR to target a piece of DNA in a mutated cell and replace it with a corrected copy right in their own Petri dish,” said Dr. Kmiec. The cell changes color if the experiment is done correctly in what promises to be another highly useful tool for educating a whole new generation of genomic engineers.

About Dr. Kmiec
Eric Kmiec, Ph.D., is widely recognized for his trailblazing work in molecular medicine and gene editing. He joins Christiana Care as director of the Gene Editing Institute he founded, now installed at the Helen F. Graham Cancer Center & Research Institute’s Center for Translational Cancer Research (CTCR).
For more than a quarter century, Dr. Kmiec has led research teams studying the reaction mechanics, biochemistry and molecular genetics of gene editing in human cells. In the late 1990s, his lab began a long-term investigation centered on understanding the mechanism and regulation of gene editing using single-stranded DNA oligonucleotides (ssODNs). The lab was a pioneering force in developing the use of these specialized ODNs for the treatment of inherited disorders.
Dr. Kmiec has been the primary mentor and Ph.D. thesis advisor for 18 doctoral students, all of whom have successful careers within the discipline. Dr. Julia Engstrom, who received a Ph.D. from the Kmiec lab in 2008, was recently appointed director of Scientific Affairs for Roche. The generation of new scientists who develop successful careers is a well-accepted, credible metric of the quality of his research program.
Dr. Kmiec founded the Gene Editing Institute to build the next generation of genetic tools for gene editing and to provide instruction in the design and implementation of these tools for collaborators and colleagues nationwide. He also founded two biotechnology companies, including OrphageniX Inc. in Delaware, which focuses on gene editing in inherited diseases such as sickle cell disease. His first company, Kimeragen, was the first gene editing company ever built and now forms part of Cibus, located in San Diego, Calif. Most recently, he is senior scientific adviser to ETAGEN, in Cambridge, Mass., where he also sits on the scientific advisory board to explore development of therapeutic uses for gene editing.
Throughout his career, Dr. Kmiec has received many research awards from the National Institutes of Health (RO1s, R21s), the American Cancer Society and private foundations, including the 2012 Proudford Foundation Unsung Hero Award in Sickle Cell Disease. He was honored as the eminent scholar in residence at Marshall University (Huntington, West Virginia) in 2009 – 2010. He has authored more than 145 scientific publications, and he holds 18 issued and licensed patents. He sits on numerous editorial and review boards.
Dr. Kmiec is a graduate of Rutgers University (1978), with a master’s degree in cell biology and biochemistry from Southern Illinois University (1980). He holds a Ph.D. in molecular biology and biochemistry from the University of Florida, Gainesville (1984). He has held numerous administrative and academic posts, most recently as professor and chair of the Department of Chemistry at Delaware State University, and served as lead on various NIH regional and state biomedical research grants, including INBRE and COBRE. He is currently an affiliated professor with the University of Delaware College of Health Sciences.