As the COVID-19 pandemic and a defining call for racial and social justice converge, researchers at ChristianaCare are working to address both. With the Broad Institute of MIT, ChristianaCare researchers are evaluating the CRISPR-based SHERLOCK COVID-19 test to ensure greater accessibility of breakthroughs to underserved populations.

In a recent op-ed, “Potential Inequities in New Medical Technologies” for Scientific American, co-written by Eric Kmiec, Ph.D, director of the Gene Editing Institute at the Helen F. Graham Cancer Center & Research Institute, the authors posed an important question: “Will new treatments continue or even worsen deeply rooted disparities? Or will we lay the groundwork for future treatments that benefit all people equitably?” Kmiec co-authored the piece with Jonathan Maron, M.D., MPH, of Dana-Farber/Boston Children’s Cancer and Blood Disorders Center.
“The scientific community is committed to fairness and inclusion in its quest for innovations that can impact care,” the authors wrote. “In working to fight COVID-19, it is critical to ensure that any breakthroughs in testing or treatment get equitably distributed.”
National projects to propel COVID-19 testing
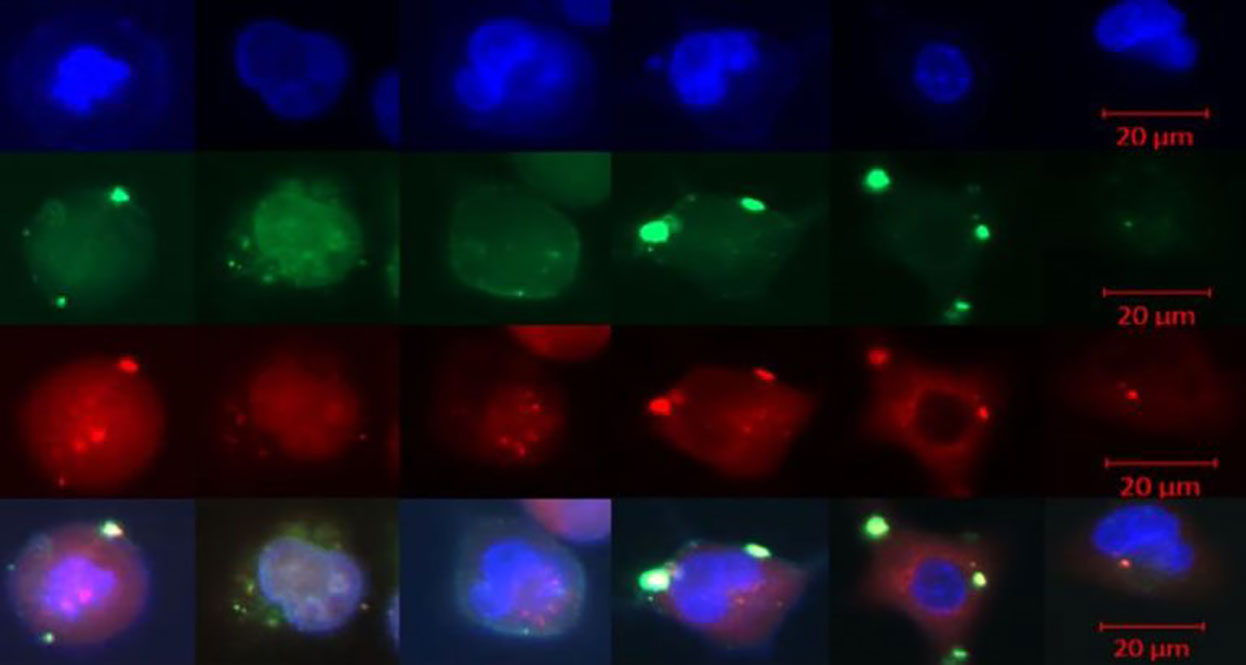
At ChristianaCare, Kmiec and colleague Brett Sansbury, Ph.D., have been helping in nationwide efforts to use the gene editing technology CRISPR to combat the COVID-19 pandemic. One project centers on the diagnostic test named SHERLOCK, short for Specific High-sensitivity Enzymatic Reporter unLOCKing, a Cas13a-based CRISPR system that targets RNA rather than DNA. The test looks for an RNA sequence specific to SARS-CoV-2, the virus that causes COVID-19, in patient samples. They are also testing other uses of CRISPR to identify human genes linked to severity of infection.
Both projects are led by Sansbury, a rising star in the world of CRISPR research and development. The Gene Editing Institute is collaborating with other research institutions through a consortium to share data and help identify susceptibility features of the virus.
Partnering with state diagnostic efforts

Sansbury and her team are using their extensive knowledge of CRISPR gene editing tools to advise Delaware public health officials on the potential of the SHERLOCK test, given emergency use authorization in May by the Food and Drug Administration.
“We’re realizing that CRISPR can offer significant advantages that allow us to capture more accurate data with fewer errors than with standard COVID-19 tests,” Sansbury said. “Our aim is to be better prepared to address subsequent outbreaks of the virus, or of new similar viruses, with the ultimate goal of developing a Delaware-specific test based on SHERLOCK that we can bring to all communities, including the underserved, in the state.”
Due to the Gene Editing Institute’s success in developing new ways of using CRISPR to rapidly locate specific mutations, it has been identified as an ideal partner for CRISPR-related COVID-19 initiatives.
One of the key drivers of the push to bring quick, accurate testing for COVID-19 via CRISPR is the accessibility and ease of use that the SHERLOCK test offers. Because it is saliva-based, the test is easier to administer than other tests, and provides results within about an hour in a format similar to a color-coded pregnancy test, making it easier to distribute and test in the community.
“The use of CRISPR will help us better respond to the next wave of the pandemic by assisting us in our testing and inclusion efforts,” Sansbury said.