In a historic next step in the development of a clinical trial for lung cancer patients using CRISPR gene editing technology, Christiana Care’s Gene Editing Institute is preparing its preliminary investigational new drug application (IND) with the U.S. Food and Drug Administration. This marks the first time Christiana Care’s Gene Editing Institute is developing its own investigator-initiated clinical trial for FDA approval.
For this inaugural clinical trial protocol, the Gene Editing Institute will use the CRISPR genome editing platform to improve the effectiveness of chemotherapy for treating K-ras-positive non-small-cell lung cancer. Patients with this disease have such a strong resistance to chemotherapy that even patients not yet treated for their cancer are resistant to it. Other patients become resistant to chemotherapy after they have been treated, which can lead to a worsening of their cancer.
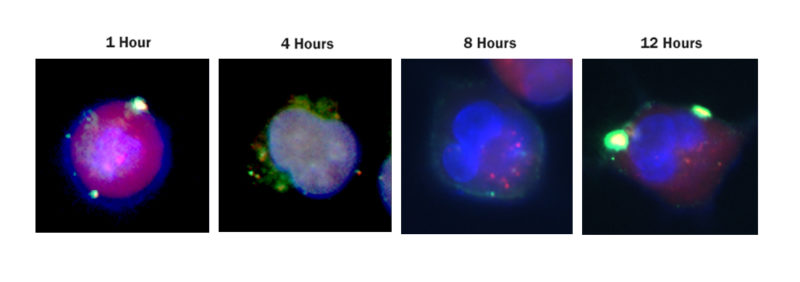
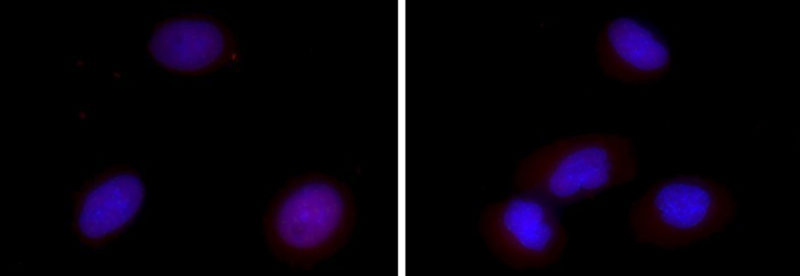
Researchers from the Gene Editing Institute published a proof of concept paper for this CRISPR approach in Molecular Therapy – Oncolytics in December 2018. In the experiment, they used CRISPR-Cas9 to disable the Nuclear Factor Erythroid 2-Related Factor (NRF2) gene in lung cancer cells by disrupting the NRF2 nuclear export signal. In tissue culture, cells lacking this gene were found to proliferate at a slower rate and be more sensitive to chemotherapeutic agents, such as cisplatin and carboplatin.
Improving lives a priority
“While we all hope for cures, the pioneering work of our gene editing team is making it a priority to improve the lives of today’s patients who are suffering with cancer and other diseases,” said Janice E. Nevin, M.D., MPH, president and CEO of Christiana Care. “Our work in this promising frontier of medicine is guided strongly by our values of love and excellence.”
“This exciting study represents a trio of firsts,” said Nicholas J. Petrelli, M.D., FACS, Bank of America endowed medical director of the Helen F. Graham Cancer Center & Research Institute at Christiana Care. “It will be the first lung cancer clinical trial carried out by a gene editing program embedded in a community cancer center. While our Gene Editing Institute has a great deal of translational research experience, this will be its first study to move scientific discovery toward an IND application. And, it will also be the first time that we will be investigating technology that was grown organically at the Gene Editing Institute.”
Ready for next step
“We are confident that the results of our animal studies now enable us to take the next step in the FDA approval process for a cancer clinical trial using CRISPR,” said Eric Kmiec, Ph.D., director of Christiana Care’s Gene Editing Institute. “It is an exciting journey. Our effort positions the Gene Editing Institute as a leader in bringing this heralded technology to people who need it most. Lots of translational research scientists are providing advice on this genetic target. Once we get to a clinical trial, our results could have a major impact on our patients, and patients around the country and world.”
The thoracic multidisciplinary team at the Graham Cancer Center & Research Institute has collaborated with the Gene Editing Institute on the clinical protocol.
“As a medical oncologist who provides care for patients with cancer, I see the devastating effects of worsening lung cancer every day,” said Gregory Masters, M.D., lung specialist at the Graham Cancer Center. “Anything we can do to lessen this burden on patients and improve their prognosis will be an important step forward.”
The Gene Editing Institute will work closely with Certara®’s highly-regarded regulatory science division, Synchrogenix, in its IND application with the FDA.
“Certara brings considerable expertise to this process and we are delighted to have them on board,” said Dr. Kmiec. The company has authored more than 20 IND applications in the past two years for both small molecules and biologics across a broad range of therapeutic areas and indications.
“We are proud to align with Christiana Care’s Gene Editing Institute on this landmark research,” said Synchrogenix President Justin Edge. “This therapeutic innovation holds great promise for correcting mutations at the precise location in the genome to treat genetic diseases and for disabling genes responsible for resistance to standard therapy.”



